Large solid pelvic mass: Think beyond fibroid

To elaborate on the title of this article, we discuss two interesting cases here.
Case-1: A 45-year old female presented for pelvic ultrasound with complaint of a swelling at the vaginal introitus. On local examination, the appearance was as shown below.
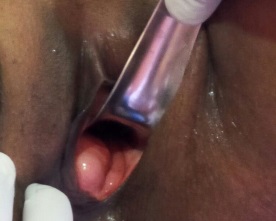
Figure 1A: Local examination reveals a focal swelling at the vaginal introitus.
The ultrasound findings in this patient were as follows:
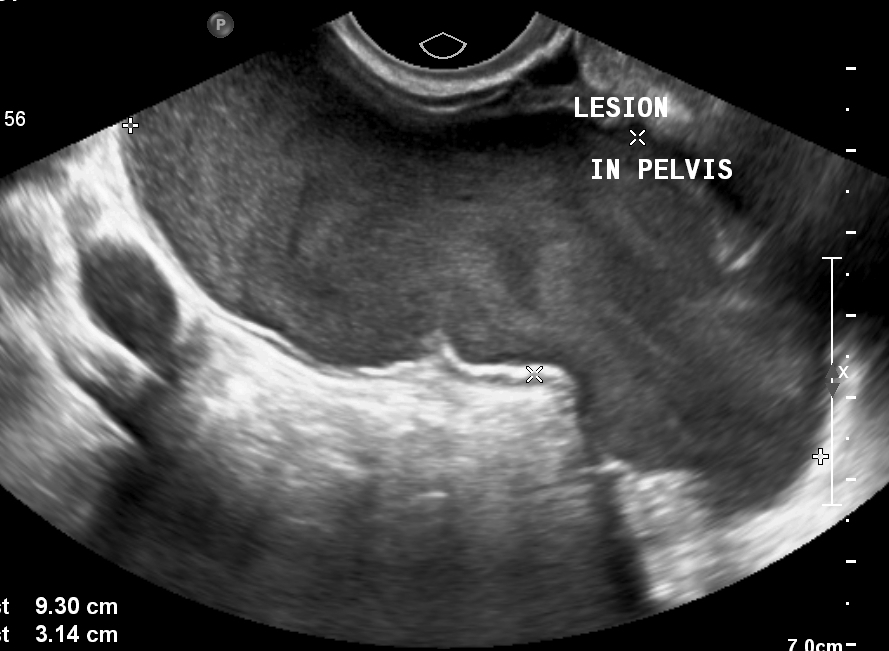
Figure 1B – Video: Transabdominal ultrasound (TAUS) shows a well-defined, lobulated hypoechoic solid mass lesion with a tiny cystic area and few echogenic linear areas, measuring 9.7 x 7.1 x 5.8 cm. The lesion is situated posterior to the uterus, closely abutting the posterior uterine & cervical wall. The mass shows smooth contour, without any evidence of calcification. Both ovaries are seen separately.
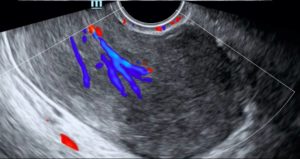
Figure 1C: Transvaginal ultrasound (TVUS) confirms the TAUS findings and additionally demonstrates internal vascularity within the lesion.
CT scan and MRI were performed, and the findings were as follows:
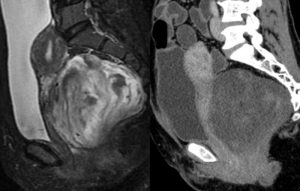
Figure 1D: The CT reveals a hypodense mass in the rectovaginal space, closely abutting the posterior wall of uterus, cervix and vagina. Few areas isodense with myometrium are seen within the lesion. The MRI (PDFS) shows a heterogeneous lesion with mixed hyper- and hypo-intense areas. Mild heterogeneous enhancement was seen on post-contrast images (not shown here).
Case 2: A 41-years old female presented for ultrasound with complaint of distension of lower abdomen. On inspection, the abdomen appeared as shown below.
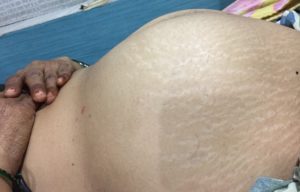
Figure 2A: On inspection, the lower abdomen appears distended.
The ultrasound findings in this patient were as follows:
Figure 2B – Video: TAUS shows a well-defined, dumbbell-shaped echogenic solid mass with intrinsic multiple cystic areas, measuring 11.3 x 9.7 x 6.4 cm in size. It is seen located posterior to the uterus, closely abutting the posterior uterine & cervical wall. The mass shows smooth contour, without any evidence of calcification. Both ovaries were seen separately (not shown here).
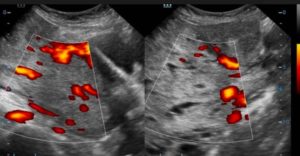
Figure 2C: TVUS confirms the TAUS findings and additionally demonstrates internal vascularity within the lesion.
MRI was performed and the findings were as follows:
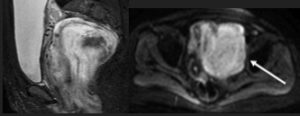
Figure 2D: The MRI (PDFS) shows a predominantly hyperintense lesion with very few hypointense areas. The mass is seen closely abutting and displacing the uterus & cervix. Moderate heterogeneous enhancement was seen on post-contrast images (not shown here).
There are few similarities in the imaging findings in both the above cases. In both the cases, differential diagnosis of subserosal fibroid & presacral schwannoma was considered, with sarcoma as less likely possibility.
TVUS-guided core biopsy was performed in both the cases, one of which has been shown below for the purpose of demonstration.
Figure 3: TVUS-guided core biopsy in Case 1. Note the elasticity of the mass, revealed by the fact that tissue surrounding the needle tract gets pulled when the needle is retracted from the lesion.
The histopathological examination revealed as follows:
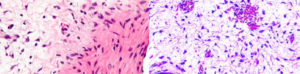
Figure 4: The histopathology from Case-1 showed mixed hypocellular and hypercellular areas. The hypocellular areas were myxoid, whereas the hypercellular areas showed stromal cells congregated around small to medium-sized blood vessels. Findings suggestive of angiomyofibroblastoma. (Image courtesy: Dr. Pratap Patil, Consultant Pathologist)
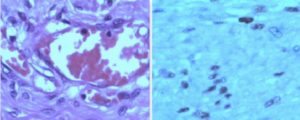
Figure 5: The histopathology from Case-2 showed spindle cells without atypia on a myxoid background, and accompanying prominent, dilated vascular channels. Findings suggestive of angiomyxoma. (Image courtesy: Dr. Chandrashekhar Bhale, Consultant Pathologist)
So, as described above, the final histopathological diagnosis was – Case-1: Angiomyofibroblastoma (AMFB); Case-2: Angiomyxoma (AMA) .
Both these lesions are rare with only few hundred cases reported worldwide. They commonly occur in premenopausal and perimenopausal women. The commonest site is vulva; however, very rarely they may present as deep-seated large pelvic masses.[1]
AMA is a locally aggressive lesion with high risk of recurrence.[2] Conversely, angiomyofibroblastoma is an indolent tumor that rarely recurs.[2] Though imaging features of AMA and AMFB may overlap, typically on ultrasound AMFB is a well-defined lesion with inhomogeneous echogenicity (multiple hypoechoic areas in echogenic stroma), whereas AMA appears as a hypoechoic mass with intrinsic echogenic septae-like areas, giving a swirled appearance, and may show cystic changes.[3],[4] AMA is much more vascular than AMFB.
The differentiation between these two tumors is made more reliably on histopathology, as described above. Both these tumors have been shown to have desmin, estrogen and progesterone receptors on immunohistochemistry, and hence that doesn’t really help differentiating between the two.
Complete surgical excision is treatment of choice for both these tumors. Patients with AMA need to be followed-up for the possibility of recurrence.
References:
1. Joseph S, Helm JM, Villegas E et al. Aggressive angiomyxoma: a rare cause of a vulvar mass. J Med Oncl Ther 2016;1(2):81-83.
2. Qiu P, Wang Z, Li Y et al. Giant pelvic angiomyofibroblastoma: a case report and review of literature. Diagn Pathol 2014;9:106.
3. Zhao CY, Su N, Jiang YX et al. Application of ultrasound in aggressive angiomyxoma: eight case reports and review of literature. World J Clin Cases 2018;6(14):811-19.
4. Rolston R, Palmer S, Ozel B. Vaginal Angiomyofibroblastoma: a case report and review of diagnostic imaging. Case Rep Obstet Gynecol 2018. Article ID 7397121. https://doi.org/10.1155/2018/7397121.