An unusual case of a tubular cystic lesion in the pelvis

A 28-years old female presented with pain in lower abdomen since 5-6 months. She had intermittent nausea and vomiting. Her menstrual cycles were regular and a recently performed urine pregnancy test was negative. Her vitals were stable and laboratory parameters were normal. A previous ultrasound report had findings suggestive of left pyosalpinx.
As a second opinion, ultrasound of abdomen and pelvis was performed again by us. The findings were as follows –
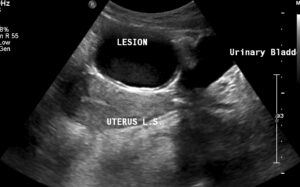
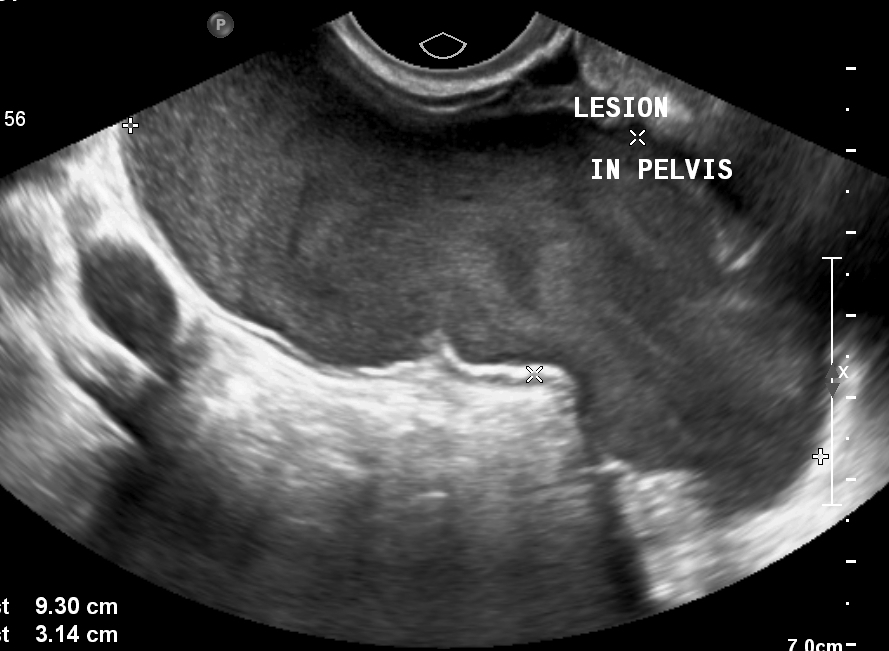
Figure 1: Transabdominal ultrasound shows a thick-walled, tubular cystic structure seen anterior to the uterus in the midline.
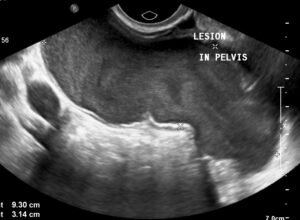
Figure 2: On transvaginal ultrasound, the cystic lesion measured approximately 9 cm x 6 cm x 3 cm. Internal echoes were noted in the fluid.
Figure 3 – Video: Transvaginal ultrasound examination reveals peristaltic movements in the pelvic lesion. Gut signature was seen in the wall of the lesion.
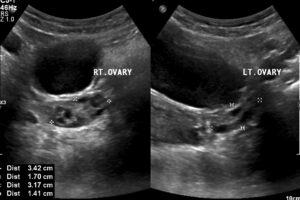
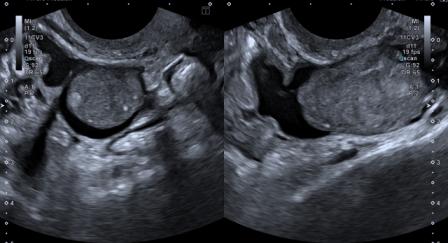
Figure 4: Both ovaries were seen separately and appeared normal.
Based on the above findings, a diagnostic possibility of enteric duplication cyst rather than pyosalpinx was suggested.
An MRI scan was performed which also revealed the tubular cystic lesion in the pelvic midline. Hydrosalpinx was suggested as a more likely diagnostic possibility as compared to cystic lesion of mesenteric origin on the MRI.
Exploratory laparotomy revealed the lesion to be an enteric duplication cyst along the terminal ileum. It was resected and ileo-colic anastomosis was performed. Histopathology confirmed the diagnosis.
Alimentary canal duplication, also known as enteric duplication cyst (EDC), is a rare congenital anomaly that can occur anywhere along the alimentary canal from the mouth to rectum. As defined by Mellish and Koop, EDCs are spherical or tubular structures that possess the characteristic mucosa of the alimentary canal supported by muscular and serous layers.[1]
The exact mechanism of this anomaly is unclear. Various theories have been proposed which suggest embryological aberrations between 4th and 8th week of gestation.[1],[2] The recanalization theory postulates a defect in the recanalization of the intestinal lumen after the solid stage of embryological development. The split notochord theory suggests defective separation of the gut endoderm and neural tube ectoderm, which may explain the association of EDC with vertebral and spinal cord anomalies. The partial twinning theory explains the association between colorectal and genitourinary duplications. Persistent embryonic diverticula theory has also been hypothesized.
Approximately 75% of EDCs are reported in the abdomen.[3] The commonest site of occurrence of EDC is the jejunoileal region, followed by esophagus.[1],[2] They are mostly solitary. Majority (80%) of EDCs are spherical cysts situated close to the gut but do not communicate with the gut lumen.[1],[2] About 20% EDCs are tubular cysts situated parallel to the gut and communicating with the lumen.[2] EDCs share a common wall with the adjacent gut but the mucosal lining is separate and may show ectopic gastric mucosa or pancreatic tissue.
The clinical presentation depends upon the location, size and type of EDC.[3] Majority (85%) cases present within the first 2 years of life, but they can present in adulthood also. Small bowel duplications may present with abdominal pain, palpable mass, nausea and vomiting. They may also present with intestinal obstruction and bleeding.
The characteristic features of small bowel EDC on ultrasound (US) are a cystic lesion situated close to the intestine with presence of internal echoes and a peristaltic double-layered wall (gut signature).[1],[2] The double-layered appearance of wall is due to the inner echogenic mucosal layer and outer hypoechoic smooth muscle layer. However, caution must be exercised as a double-layered appearance of wall has also been rarely described in ovarian and mesenteric cysts.[2] New, specific signs such as a five-layered wall and Y-configuration of the shared muscularis propria have been described in EDC. Detecting these signs requires high-resolution US probe and greater expertise.[2] Peristalsis is, however, a classic US feature.[1],[2]
Computerised Tomography (CT) and MRI have a role in establishing the exact location of the cyst and its relationship with adjacent structures.[3] Where the US diagnosis is not clear, CT / MRI can help characterise the lesion.
The common surgical approach is resection of the cyst with primary anastomosis.[1] Complete resection is important as there are chances of recurrence and malignant changes.[2]
© Copyright Reserved
References:
1. Martini C, Pagano P, Perrone G, Bresciani P, Dell’Abate P. Intestinal duplications: incidentally ileum duplication cyst in young female. BJR Case Rep. 2019;5(3):20180077. Published 2019 Apr 3. doi:10.1259/bjrcr.20180077
2. Sangüesa Nebot C, Llorens Salvador R, Carazo Palacios E, Picó Aliaga S, Ibañez Pradas V. Enteric duplication cysts in children: varied presentations, varied imaging findings. Insights Imaging. 2018;9(6):1097-1106. doi:10.1007/s13244-018-0660-z
3. Sharma S, Yadav AK, Mandal AK, Zaheer S, Yadav DK, Samie A. Enteric Duplication Cysts in Children: A Clinicopathological Dilemma. J Clin Diagn Res. 2015;9(8):EC08-EC11. doi:10.7860/JCDR/2015/12929.6381